Iba1抗体,兔源(免疫组化用)
Anti Iba1, Rabbit (for Immunocytochemistry)
- 产品特性
- 相关资料
- Q&A
- 参考文献
Iba1抗体,兔源(免疫组化用)
Anti Iba1, Rabbit (for Immunocytochemistry)
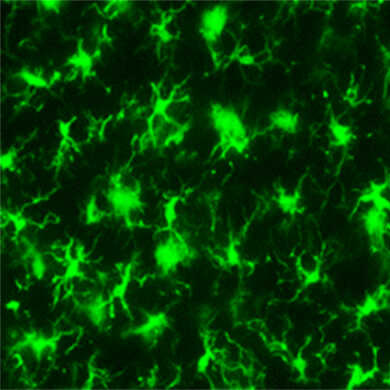
Iba1(Ionized calcium-binding adapter molecule 1)是分子量约为17 kDa的钙结合蛋白。由于在中枢神经系统中,Iba1在小胶质细胞中特异性表达1),因此被用作小胶质细胞标志物。据报告称,Iba1在静息型小胶质细胞和活化型小胶质细胞中均有表达,但有报告称在活化型小胶质细胞中其表达会有所增加2)。另外,Iba1也会在外周组织的巨噬细胞中表达,被称为AIF-1(同种异体移植物炎症因子1,Allograft inflammatory factor-1)。Iba1在细胞内与F-肌动蛋白结合,发挥着形成肌动蛋白纤维束的作用。这种肌动蛋白纤维束的形成被认为是细胞迁移和吞噬过程中观察细胞膜波动结构形成所必须的3)。
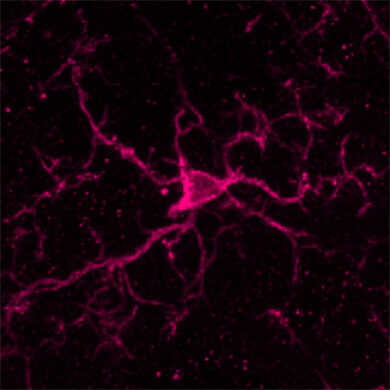
抗Iba1,兔源(免疫组化用)在免疫组织染色中可以对小胶质细胞包括突起一并进行染色,因此被世界各地的研究人员作为小胶质细胞标记抗体的标准来使用。
近年来,小胶质细胞因其神经营养和保护作用以及通过产生NO、TNF-α和IL-1β引起的神经损伤作用备受关注。
小胶质细胞的染色图像
Anti Iba1,兔源(免疫组化用)
◆特点
● 在免疫组织染色(冰冻切片)中可染色小胶质细胞包括其突起※
● 使用浓度为1︰500 – 1︰1,000,少量使用也可染色。
※ 染色程度取决于样品状况,无法保证一定能染色成功。
◆抗体信息
|
抗体种类 |
多克隆抗体 |
|
抗原 |
Iba1的合成肽(C端序列相同) |
|
免疫动物 |
兔子 |
|
交叉性 |
人、小鼠、大鼠、其他※ |
|
浓度 |
0.5-0.7 mg/mL |
|
应用 |
免疫组织染色(冰冻切片):1︰500-1,000 免疫细胞染色: 1︰500-1,000 |
|
储存条件 |
-20℃冷冻保存(干冰运输) |
|
抗原来源动物 |
大鼠 |
※ 拥有犬4)、猫5)、猪6)、狨猴7)、斑马鱼8)的应用实例
点击此处查看使用论文
◆应用数据
免疫组织染色(小鼠)
|
|
物种:小鼠 部位:小脑 样品:冰冻切片 抗体浓度:1︰1,000 |
|
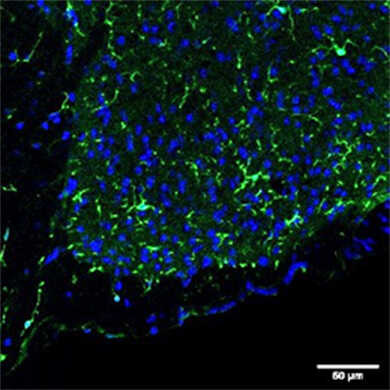
物种:小鼠 部位:伏隔核 样品:超薄切片 抗体浓度:1︰500 <数据提供> 东京理科大学生命生物科学科 萩原老师、山崎先生 |
|
|
物种:小鼠 部位:脊髓 样品:冰冻切片 抗体浓度:1︰500 <数据提供> 东京理科大学生命生物科学科 萩原老师、山崎先生 |
|
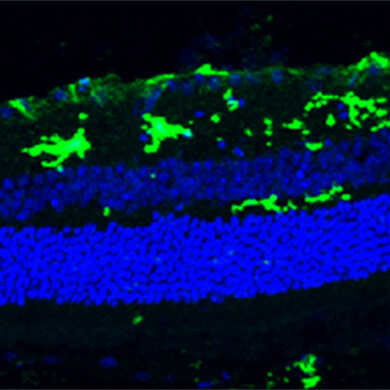
物种:小鼠 部位:视网膜 抗体浓度:1︰1,000 <数据提供> Prof. Lieve Moons, KU Leuven, Belgium. |
免疫组织染色(大鼠)
|
|
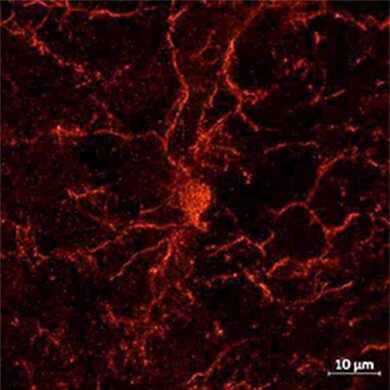
物种:大鼠 部位:大脑皮层 样品:冰冻切片 抗体浓度:1︰1,000 <数据提供> 日本国立精神·神经医疗研究中心 佐柳老师、真锅老师、一户老师、高坂老师 |
◆Anti Iba1抗体系列
|
产品详情 |
产品等级 |
|
小胶质细胞标记物抗体——Anti Iba1抗体 |
免疫化学用 |
|
抗Iba1, 重组兔源单克隆抗体(6A4) |
免疫化学用 |
|
抗Iba1,兔(免疫细胞化学用) |
免疫化学用 |
|
抗Iba1,兔(石蜡切片用) |
免疫化学用 |
|
抗Iba1,兔 生物素结合 |
免疫化学用 |
|
抗Iba1,兔源(结合488绿色荧光)(Prototype) |
免疫化学用 |
|
抗Iba1, 兔源, 结合SPICA Dye™ 568 |
免疫化学用 |
|
抗 Iba1,兔源,SPICA Dye™ 594偶联 |
免疫化学用 |
|
Anti-Iba1、红色荧光染料(635)结合 |
免疫化学用 |
|
抗Iba1,山羊多克隆抗体 |
免疫化学用 |
|
鼠源Iba1抗体,无标签,单克隆抗体(NCNP24) |
免疫化学用 |
|
抗Iba1, 兔源单克隆抗体(NCNP27) |
免疫化学用 |
相关资料
◆原代小胶质细胞ICC实验方案示例
将从新生大鼠(出生0~2天)的大脑中制备和培养的原代混合胶质细胞按照1.0×105 cell/well接种至4 well-plate、13 φmm盖玻片(PLL涂层)。
↓
固定:4% formaldehyde-PBS, 15 min
↓
清洗:PBS
↓
通透:0.1% TX-100 PBS, 5 min
↓
封闭:3% BSA-3% normal goat serum-PBS (blocking buffer), >15min
↓
一抗反应:抗Iba1抗体(1:1,000)blocking buffer, 1h
↓
清洗
↓
二抗反应:Alexa Fluor 488标记抗兔IgG抗体/blocking buffer, 1h
↓
封片
↓
在荧光显微镜下进行观察

小胶质细胞产品目录
FAQ
◆关于抗体
Q1:抗原是什么样的?
A1:是Iba1的合成肽(C端序列相同)。具体序列未公开。
Q2:50 μg的使用量是多少?
A2:在免疫组织染色中,每张玻片使用200 μL抗体溶液(稀释度为 1:1,000)的情况下,可使用约500次。
◆关于实验方案
Q3:建议使用哪种二抗?
A3:FUJIFILM Wako拥有以下二抗的使用实例,可供参考。
Alexa Fluor® 488-AffiniPure Goat Anti-Rabbit IgG (H+L) (Jackson ImmunoResearch公司, 厂家编号: 111-545-144)
Alexa Fluor® 647-conjugated AffiniPure Donkey anti-Rabbit IgG (H+L) (Jackson ImmunoResearch公司, 厂家编号: 711-605-152)
◆关于故障排除
Q4:WB中无法检测出条带
A4:本抗体适用于免疫组织染色(冰冻切片)以及免疫细胞染色,如需进行WB,请使用 抗Iba1,兔源(Western Blotting用) (产品编号:016-20001)
Q5:免疫组织染色和免疫细胞染色中小胶质细胞无法染色
A5:可能导致此问题的原因有以下几种,请尝试对应的解决方案。
1. 固定不充分
有报告称,未灌注固定或固定不充分的样品染色效果会降低。请在免疫组织染色中使用4%多聚甲醛(PFA)进行灌注固定后再制备冰冻切片。
2. 抗原变性
请尝试在以下条件下进行抗原修复操作
(A)柠檬酸缓冲液(pH 6.0), 90℃, 9 min
(B)TE缓冲液(pH 9.0), 90℃, 9 min
3. 样品变质
需要重新制备样品,切片厚度约为20-50 μm。
4. 抗体量少
请提高抗体浓度,推荐浓度为1︰500~1,000。
Q6:免疫组织染色和免疫细胞染色的背景过高。
A6:导致此问题的可能原因有以下几种,请尝试对应的解决方案。
① 封闭不充分
请尝试延长封闭的孵育时间或更换封闭试剂。建议使用推荐Protocol中记载的1% BSA, 含0.3% TritonX-100的PBS和含1% BSA, 0.3% Tween-20的PBS,或3%二抗宿主的正常血清。
② 一抗的量过多
请降低一抗浓度,推荐浓度为1︰500~1,000。
③ 二抗的反应时间过长
请缩短反应时间。推荐反应时间为1~2 h,或增加二抗反应后的清洗次数。
④ 样品变质
需要重新制备样品,切片厚度约为20-50 μm。
⑤ 抗原变性
请尝试在以下条件下进行抗原修复操作
(A)柠檬酸缓冲液(pH 6.0), 90℃, 9 min
(B)TE缓冲液(pH 9.0), 90℃, 9 min
⑥ 内源性的过氧化物酶发生反应(使用HRP或POD作为检测酶时)
为了灭活内源性过氧化物酶,请在阻断前使用含3%过氧化氢的80%甲醇溶液,并在-20℃条件下处理20 min。
Q7:在免疫组织染色中,除小胶质细胞外,神经细胞也被染色。
A7:可能导致此问题的原因有以下几种,请尝试对应的解决方案。
① 抗体的量过多
请降低一抗或二抗的抗体浓度,抗Iba1抗体的推荐浓度为1︰500~1,000。
② 二抗的反应时间过长
请缩短反应时间。推荐反应时间为1~2 h,或增加二抗反应后的清洗次数。
③ 抗原变性
请尝试在以下条件下进行抗原修复操作
(A)柠檬酸缓冲液(pH 6.0), 90℃, 9 min
(B)TE缓冲液(pH 9.0), 90℃, 9 min
◆关于应用数据
Q8:本抗体可以用于流式细胞仪吗?
A8:FUJIFILM Wako没有使用实例,但以下论文中使用了本抗体,敬请参考。
Koh, H. S., et al.: Nat. Commun., 6(1),1(2015).
The HIF-1/glial TIM-3 axis controls inflammation-associated brain damage under hypoxia.
参考文献
|
1. |
Imai, Y., Ibata, I., Ito, D., Ohsawa, K., & Kohsaka, S.: Biochemical and biophysical research communications, 224(3), 855(1996). A Novel Geneiba1 in the Major Histocompatibility Complex Class III Region Encoding an EF Hand Protein Expressed in a Monocytic Lineage |
|
2. |
Mori, I., Imai, Y., Kohsaka, S., & Kimura, Y.: Microbiology and immunology, 44(8), 729(2000). Upregulated expression of Iba1 molecules in the central nervous system of mice in response to neurovirulent influenza A virus infection |
|
3. |
Sasaki, Y., Ohsawa, K., Kanazawa, H., Kohsaka, S., & Imai, Y.: Biochemical and biophysical research communications, 286(2), 292(2001). Iba1 is an actin-cross-linking protein in macrophages/microglia. |
|
4. |
Ahn, J.H., et al.: Lab. Anim. Res., 28(3), 165 (2012). Comparison of alpha-synuclein immunoreactivity in the spinal cord between the adult and aged beagle dog |
|
5. |
Ide, T., et al.: J. Vet. Med .Sci., 72(1), 99 (2010). Histiocytic Sarcoma in the Brain of a Cat |
|
6. |
Gaige, S., et al.: Neurotoxicology, 34, 135(2013). c-Fos immunoreactivity in the pig brain following deoxynivalenol intoxication: Focus on NUCB2/nesfatin-1 expressing neurons |
|
7. |
Rodriguez-Callejas, J.D. et al.: Front. Aging Neurosci., 8, 315(2016). Evidence of Tau Hyperphosphorylation and Dystrophic Microglia in the Common Marmoset |
|
8. |
Fantin, A., et al.: Blood, 116(5), 829(2010). |
◆Nature
|
1. |
Lam, C.K., et al.: Nature, 465, 478(2010). Embolus extravasation is an alternative mechanism for cerebral microvascular recanalization.
|
|
2. |
Stefater, J. A. 3rd. et al.: Nature, 474, 511(2011). Regulation of angiogenesis by a non-canonical Wnt-Flt1 pathway in myeloid cells.
|
|
3. |
Deng, H. X., et al.: Nature, 477, 211(2011). Mutations in UBQLN2 cause dominant X-linked juvenile and adult onset ALS and ALS/dementia. |
|
4. |
Lee, Y., et al.: Nature, 487, 433(2012). Oligodendroglia metabolically support axons and contribute to neurodegeneration. |
|
5. |
Heneka, M. T., et al.: Nature, 493, 674(2013). NLRP3 is activated in Alzheimer's disease and contributes to pathology in APP/PS1 mice.
|
|
6. |
Shao, W., et al.: Nature, 494, 90(2013). Suppression of neuroinflammation by astrocytic dopamine D2 receptors via αB-crystallin
|
|
7. |
Zhang, G., et al.: Nature, 497, 211(2013). Hypothalamic programming of systemic ageing involving IKK-β, NF-κB and GnRH
|
|
8. |
Chung, W. S., et al.: Nature, 504, 394(2013). Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways.
|
|
9. |
Roth, T. L., et al.: Nature, 505, 223(2014). Transcranial amelioration of inflammation and cell death after brain injury
|
|
10. |
Najm, F. J., et al.: Nature, 522, 216(2015). Drug-based modulation of endogenous stem cells promotes functional remyelination in vivo. |
|
11. |
Fourgeaud, L., et al.: Nature, 532, 240(2016). TAM receptors regulate multiple features of microglial physiology.
|
|
12. |
Vasek, M. J., et al.: Nature, 534, 538(2016). A complement-microglial axis drives synapse loss during virus-induced memory impairment. |
|
13. |
Iaccarino, H. F., et al.: Nature, 540, 230(2016). Gamma frequency entrainment attenuates amyloid load and modifies microglia.
|
|
14. |
Bialas, A. R. et al.: Nature, 546, 539(2017). Microglia-dependent synapse loss in type I interferon-mediated lupus
|
|
15. |
Mass, E., et al.: Nature, 549, 389(2017). A somatic mutation in erythro-myeloid progenitors causes neurodegenerative disease.
|
|
16. |
Jun, J. J., et al.: Nature, 551, 232(2017). Fully integrated silicon probes for high-density recording of neural activity.
|
|
17. |
Bussian, T. J., et al.: Nature, 562, 578(2018). |
◆CELL
|
1. |
Lujambio, A., et al.: Cell, 153, 2, 449(2013). Non-Cell-Autonomous Tumor Suppression by p53.
|
|
2. |
Parkhurst, C. N., et al.: Cell, 155, 7, 1596(2013). Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor.
|
|
3. |
Wang, Y., et al.: Cell, 160, 6, 1061(2015). TREM2 Lipid Sensing Sustains the Microglial Response in an Alzheimer’s Disease Model.
|
|
4. |
Keren-Shaul, H., et al.: Cell, 169, 7, 1276(2017). A Unique Microglia Type Associated with Restricting Development of Alzheimer's Disease.
|
|
5. |
Ulland, T. K., et al.: Cell, 170, 4, 649(2017). TREM2 Maintains Microglial Metabolic Fitness in Alzheimer’s Disease.
|
|
6. |
Qin, Y., et al.: Cell, 174, 1, 156(2018). A Milieu Molecule for TGF-β Required for Microglia Function in the Nervous System.
|
|
7. |
Yan, S., et al.: Cell, 173, 4, 989(2018). A Huntingtin Knockin Pig Model Recapitulates Features of Selective Neurodegeneration in Huntington's Disease |
◆Nature Medicine
|
1. |
Heppner, F. L., et al.: Nat. Med., 2, 146(2005). Experimental autoimmune encephalomyelitis repressed by microglial paralysis.
|
|
2. |
Nikić, I., et al.: Nat. Med., 4, 495(2011). A reversible form of axon damage in experimental autoimmune encephalomyelitis and multiple sclerosis. |
|
3. |
Vom, B. J., et al.: Nat. Med., 12, 1812(2012). Inhibition of IL-12/IL-23 signaling reduces Alzheimer’s disease–like pathology and cognitive decline
|
|
4. |
Minami, S. S., et al.: Nat. Med., 10, 1157(2014). Progranulin protects against amyloid β deposition and toxicity in Alzheimer's disease mouse models.
|
|
5. |
Yun, S. P., et al.: Nat. Med., 7, 931(2018). Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson's disease.
|
|
6. |
Mount, C. W., et al.: Nat Med. 5, 572(2018). Potent antitumor efficacy of anti-GD2 CAR T cells in H3-K27M+ diffuse midline gliomas.
|
◆Nature Neuroscience
|
1. |
Zhang, K., et al.: Nat. Neurosci., 10, 1064(2003). HIV-induced metalloproteinase processing of the chemokine stromal cell derived factor-1 causes neurodegeneration.
|
|
2. |
Ajami, B., et al.: Nat. Neurosci., 12, 1538(2007). Local self-renewal can sustain CNS microglia maintenance and function throughout adult life
|
|
3. |
Mildner, A., et al.: Nat. Neurosci., 12, 1544(2007). Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions.
|
|
4. |
Bero, A. W., et al.: Nat. Neurosci., 6, 750(2011). Neuronal activity regulates the regional vulnerability to amyloid-β deposition.
|
|
5. |
Fancy, S. P., et al.: Nat. Neurosci., 14, 1009(2011). Axin2 as regulatory and therapeutic target in newborn brain injury and remyelination.
|
|
6. |
Ajami, B., et al.: Nat. Neurosci., 14, 1142(2011). Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool.
|
|
7. |
Mosher, K. I. et al.: Nat. Neurosci., 11, 1485(2012). Neural progenitor cells regulate microglia functions and activity.
|
|
8. |
Lehmann, S. M., et al.: Nat. Neurosci., 6, 827(2012). An unconventional role for miRNA: let-7 activates Toll-like receptor 7 and causes neurodegeneration. |
|
9. |
Kierdorf, K., et al.: Nat. Neurosci., 3, 273(2013). Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways
|
|
10. |
Bialas, A. R. et al.: Nat. Neurosci., 12, 1773(2013). TGF-β signaling regulates neuronal C1q expression and developmental synaptic refinement
|
|
11. |
Butovsky, O., et al.: Nat. Neurosci., 1, 131(2014). Identification of a Unique TGF-β Dependent Molecular and Functional Signature in Microglia.
|
|
12. |
Saito, T., et al.: Nat. Neurosci., 5, 661(2014). Single App knock-in mouse models of Alzheimer's disease.
|
|
13. |
Erny, D., et al.: Nat. Neurosci., 7, 965(2015). Host microbiota constantly control maturation and function of microglia in the CNS. |
|
14. |
Sorge, R. E. et al.: Nat. Neurosci., 8, 1081(2015). Different immune cells mediate mechanical pain hypersensitivity in male and female mice.
|
|
15. |
Hama, H., et al.: Nat. Neurosci., 10, 1518(2015). ScaleS: an optical clearing palette for biological imaging.
|
|
16. |
Asai, H., et al.: Nat. Neurosci., 11, 1584(2015). Depletion of microglia and inhibition of exosome synthesis halt tau propagation.
|
|
17. |
Guan, Z., et al.: Nat. Neurosci., 1, 94(2016). Injured sensory neuron-derived CSF1 induces microglial proliferation and DAP12-dependent pain.
|
|
18. |
Grabert, K., et al.: Nat. Neurosci., 3, 504(2016). Microglial brain region-dependent diversity and selective regional sensitivities to aging
|
|
19. |
Gonçalves, J. T., et al.: Nat. Neurosci., 6, 788(2016). In vivo imaging of dendritic pruning in dentate granule cells
|
|
20. |
Liu, Q., et al.: Nat. Neurosci., 2, 243(2016). Neural stem cells sustain natural killer cells that dictate recovery from brain inflammation.
|
|
21. |
Safaiyan, S., et al.: Nat. Neurosci., 8, 995(2016). Age-related myelin degradation burdens the clearance function of microglia during aging.
|
|
22. |
Pandya, H., et al.: Nat. Neurosci., 5, 753(2017). Differentiation of human and murine induced pluripotent stem cells to microglia-like cells
|
|
23. |
Füger, P., et al.: Nat. Neurosci., 10, 1371(2017). Microglia turnover with aging and in an Alzheimer's model via long-term in vivo single-cell imaging |
◆Nature Immunology
|
1. |
Wang, Y., et al.: Nat. Immunol., 13, 753(2012). IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia.
|
|
2. |
Goldmann, T., et al.: Nat. Immunol., 17, 797(2016). Origin, fate and dynamics of macrophages at central nervous system interfaces |
|
3. |
Haimon, Z., et al.: Nat. Immunol., 19, 636(2018). Re-evaluating microglia expression profiles using RiboTag and cell isolation strategies. |
◆Nature Biotechnology
|
1. |
Park, S. I., et al.: Nat. Biotechnol., 33, 1280(2015). Soft, stretchable, fully implantable miniaturized optoelectronic systems for wireless optogenetics
|
|
2. |
Staahl, B. T., et al.: Nat. Biotechnol., 35, 431(2017). Efficient genome editing in the mouse brain by local delivery of engineered Cas9 ribonucleoprotein complexes |
◆Nature Methods
|
1. |
Clark, J. J., et al.: Nat. Methods., 7, 126(2010). Chronic microsensors for longitudinal, subsecond dopamine detection in behaving animals
|
|
2. |
Prevedel, R., et al.: Nat. Methods., 13, 1021(2016). |
◆Neuron
|
1. |
Simard, A. R., et al.: Neuron, 49, 4, 489(2006). Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer's disease.
|
|
2. |
Bhaskar, K., et al.: Neuron, 68, 1, 19(2010). Regulation of tau pathology by the microglial fractalkine receptor.
|
|
3. |
Bergmann, O., et al.: Neuron, 74, 4, 634(2012). The Age of Olfactory Bulb Neurons in Humans
|
|
4. |
Schafer, D. P., et al.: Neuron, 74, 4, 691(2012). Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner.
|
|
5. |
Paolicelli, R. C., et al.: Neuron, 95, 2, 297(2017). TDP-43 Depletion in Microglia Promotes Amyloid Clearance but Also Induces Synapse Loss.
|
|
6. |
Tufail, Y., et al.: Neuron, 93, 3, 574(2017). Phosphatidylserine Exposure Controls Viral Innate Immune Responses by Microglia.
|
|
7. |
Abud, E. M., et al.: Neuron, 94, 2, 278(2017). iPSC-Derived Human Microglia-like Cells to Study Neurological Diseases.
|
|
8. |
Bohlen, C. J., et al.: Neuron, 94, 4, 759(2017). Diverse Requirements for Microglial Survival, Specification, and Function Revealed by Defined-Medium Cultures.
|
|
9. |
De, Biase, L. M., et al.: Neuron, 95, 2, 341(2017). Local Cues Establish and Maintain Region-Specific Phenotypes of Basal Ganglia Microglia.
|
|
10. |
Hwang, H. W., et al.: Neuron, 95, 6, 1334(2017). cTag-PAPERCLIP Reveals Alternative Polyadenylation Promotes Cell-Type Specific Protein Diversity and Shifts Araf Isoforms with Microglia Activation.
|
|
11. |
Lehrman, E. K., et al.: Neuron, 100, 1, 120(2018). CD47 Protects Synapses from Excess Microglia-Mediated Pruning during Development.
|
|
12. |
López-Erauskin, J., et al.: Neuron, 100, 4, 816(2018). ALS/FTD-Linked Mutation in FUS Suppresses Intra-axonal Protein Synthesis and Drives Disease Without Nuclear Loss-of-Function of FUS |
| 产品编号 | 产品名称 | 产品规格 | 产品等级 | 备注 |
| 019-19741 | Anti Iba1, Rabbit (for Immunocytochemistry) 抗Iba1,兔(免疫组化用) |
50 μg | 免疫化学用 | – |
|
|